Fields of research: Anja Goldmann
Currently, Anja is working on the project titled “Living Free Radical Polymerizations for Nanotechnology Applications”. Her PhD project encompasses developing routes to the synthesis of Cyclic Polymer Brushes. This collaborative project between Prof. Christopher Barner-Kowollik (CAMD), Ass. Prof. Martina Stenzel (CAMD) and our group is supported by the DFG (Deutsche Forschungsgemeinschaft) and the ARC (Australian Research Council). It involves the synthesis of cyclic polymers by combining Reversible Addition Fragmentation Chain Transfer (RAFT) polymerization and “click-chemistry”. The novel strategy entails the synthesis of linear polymer backbones (e.g. styrene, acrylates, methacrylates,…) followed by endgroup modification to facilitate click chemistry for the formation of ring shaped polymers.
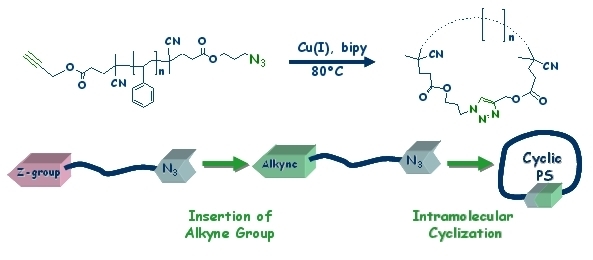
The coupling of the reversible addition fragmentation chain transfer (RAFT) polymerization technique with the copper catalyzed Huisgen 1,3-dipolar cycloaddition (click chemistry) as a simple and effective way to generate macrocycles is used. The novel strategy entails the synthesis of linear polystyrene backbones followed by endgroup modification to facilitate click chemistry for the formation of ring shaped polymers. An azido group modified 4-cyanopentanoic acid dithiobenzoate is employed as the chain transfer agent in the RAFT mediated polymerization of styrene to form the telechelic polymers. To facilitate the cyclization of the polystyrene chains by click coupling, the thiocarbonyl thio endgroup is removed and concomitantly replaced by an alkyne bearing function. This is carried out via the radical decomposition of excess azobis(4-cyano valeric acid) (ACVA) modified with an alkyne endgroup in the presence of the thiocarbonyl thio capped PS. Click-cyclization of telechelic homopolymers is acquired under high dilution. This improved method avoids the presence of thiocarbonyl thio functions in the macrocycle, thus considerably increasing the chemical stability of these polymers.


