 Dr.
Dr.Markus Burkhardt
PhD studentAt Macromolekulare Chemie II until 06/2007
e-Mail: markus.burkhardt(at)kurz.de
LEONHARD KURZ Stiftung & Co. KG
Schwabacher Straße 482
90763 Fürth/Germany
http://www.kurz.de/
Markus Burkhardt
CU 4, Entwicklungsteam Security
Entwicklung
LEONHARD KURZ Stiftung & Co. KG
Schwabacher Straße 482
90763 Fürth/Germany
Tel: +49 911 71 41 9284
Fax: +49 911 71 41 505
e-mail: markus.burkhardt@kurz.de
Different Routes of Formation of Interpolyelectrolyte Complexes (IPECs) using Amphiphilic Block Copolymers Synthesis and Characterization of Aggregates in Aqueous Solutions
M. Burkhardt1, D. Pergushov2, M. Gradzielski3, A.H.E. Müller1 (1Makromolukulare Chemie II, Universität Bayreuth; 2Moscow State University; 3Technische Universität Berlin, Stranski-Laboratorium für Physikalische Chemie und Theoretische Chemie)
A promising way to transfer PIB - synthesized by living cationic polymerization - to anionic polymerization is to endcap the growing macrocation with thiophene (T). The obtained species can be used as macroinitiator for anionic polymerization of e.g. tBMA after activation with BuLi [1]. The resulting hydrolyzed linear PIB-b-PMAA diblock copolymer has been studied in the framework of collaboration between groups from the Bayreuth and Moscow.
We also study the interaction of PIB-b-PMAA micelles with a positively charged polyelectrolyte, quaternized poly(4-vinylpyridine) (P4VPQ). It was shown that the architectures generated upon addition of the cationic polyelectrolyte to micellar solutions of PIB-b-PMAA can be described as core-shell-corona complexes (Scheme 1). PIB forms the hydrophobic core (A), which is surrounded by the condensed shell complex formed by the PMAA/P4VPQ (B). The corona consists of excess PMAA (C) and keeps the whole complex micelle in solution.
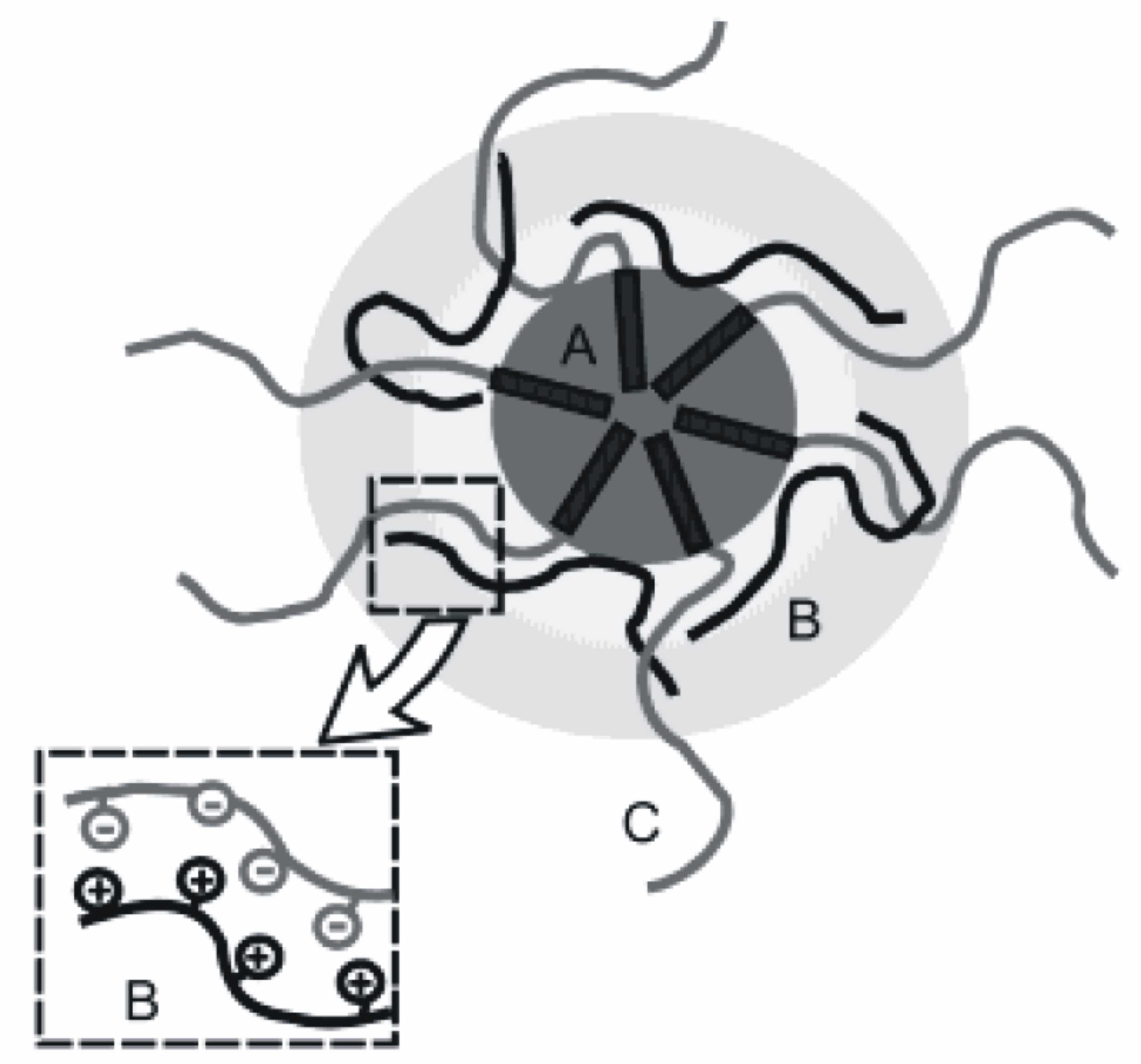
Scheme 1: IPEC with a PIB core, a complex shell, and a PMAA corona
Up to now different copolymers were synthesized. After hydrolysis of the t-butyl-groups, the polymer is water-soluble. The cmc was determined in collaboration with the Moscow state university (Dr. D. Pergushov) via following the ratio of two absorption peaks of pyrene [2] in a concentration row of block copolymer solutions in water (scheme 2). Comparing the results with formerly obtained results, a pronounced influence of the hydrophobic PIB-block can be investigated. The longer the block, the higher is the tendency to form aggregates, the lower the cmc. The influence of the PMAA-block seems to be negligible.
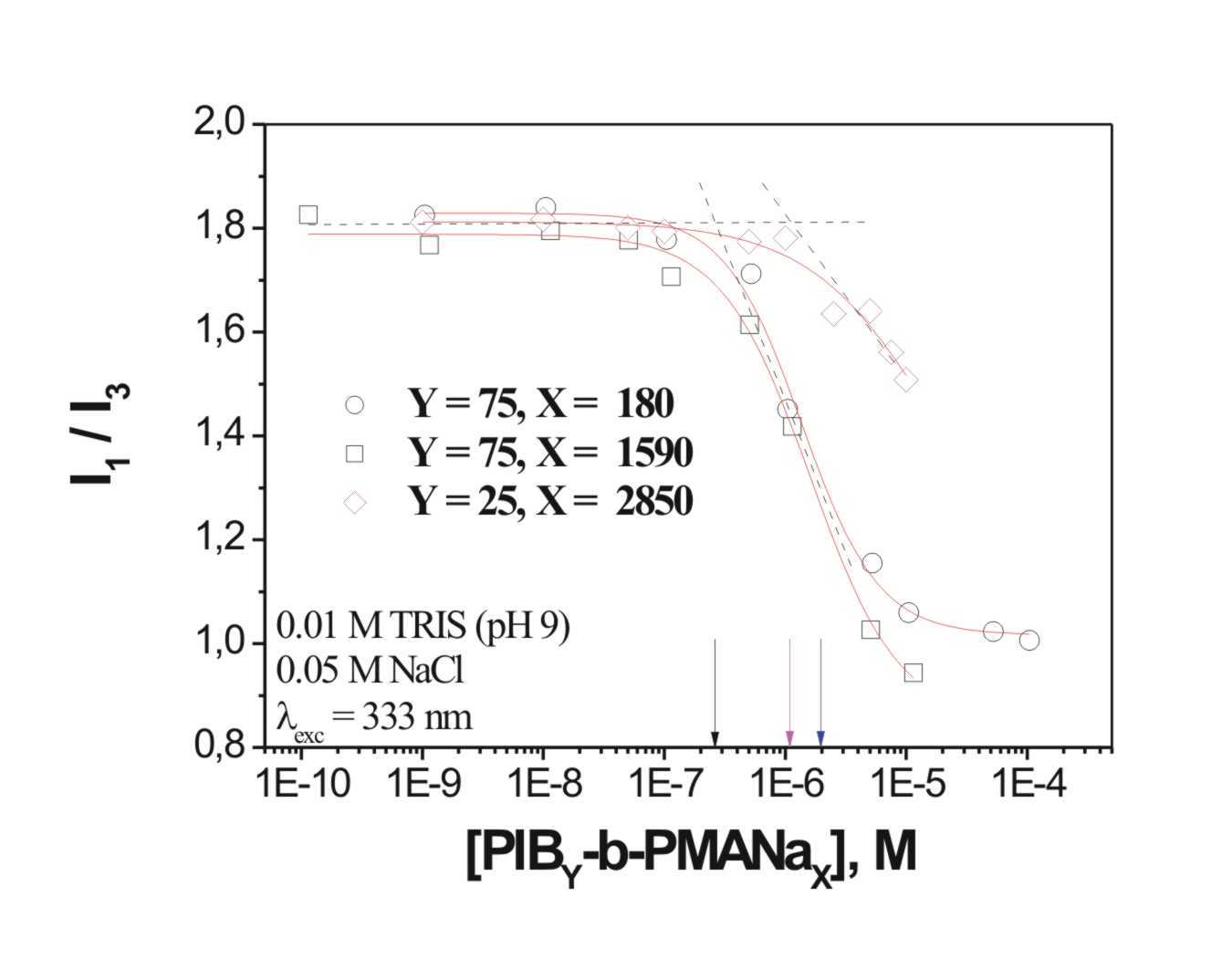
Scheme 2: Influence of PIB-blocklength on cmc
Furthermore DLS-measurements depending on a change of salt concentration and pH-value, that changes the degree of neutralization of the PMAA-blocks, revealed a big influence on the size of the aggregates. As it can be seen (scheme 3, 4), size of the micellar aggregates decreases with increasing salt concentration. This might be due to screening of the negative charges in the corona of the structures. So the arms of the micelles can be packed closer, the hydrodynamic radius increases. The same effect can be seen with changes of pH-value. The lower the pH-value is, the more uncharged COOH-groups are in the corona of the micelles, the less pronounced is the repulsion of the PMAA-blocks. As a result of this, the arms can pack closer, the Rh also decreases. The effect of salt and pH on the core of the aggregates can be followed by means of SANS. The measurements were made in Saclay/F and Grenoble/F. The latest evaluations are still under progress. The changes should be followed by the results of a fit of the curves according to a core(-shell; in case of IPECs)-corona fit of spherical particles [3].
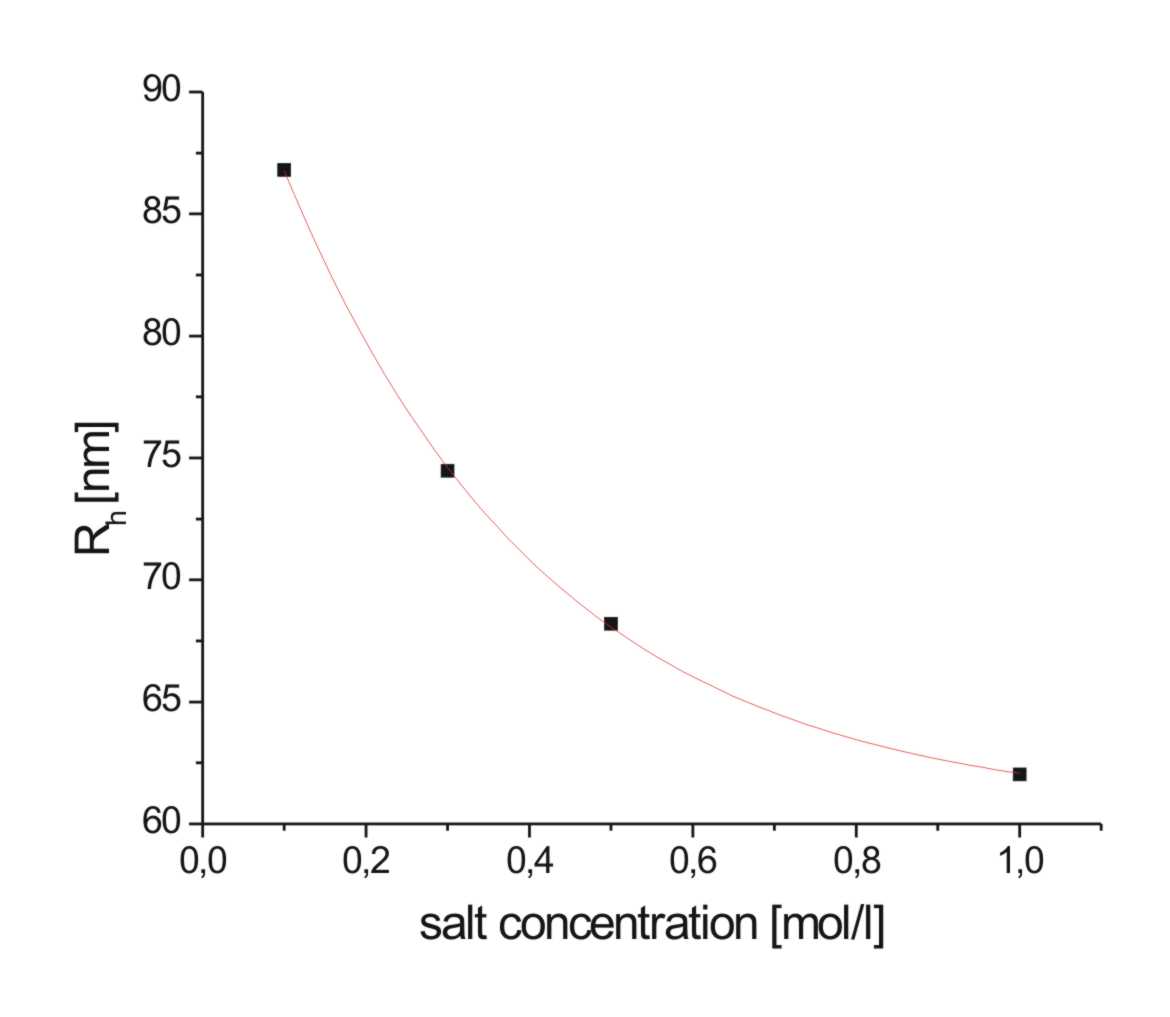
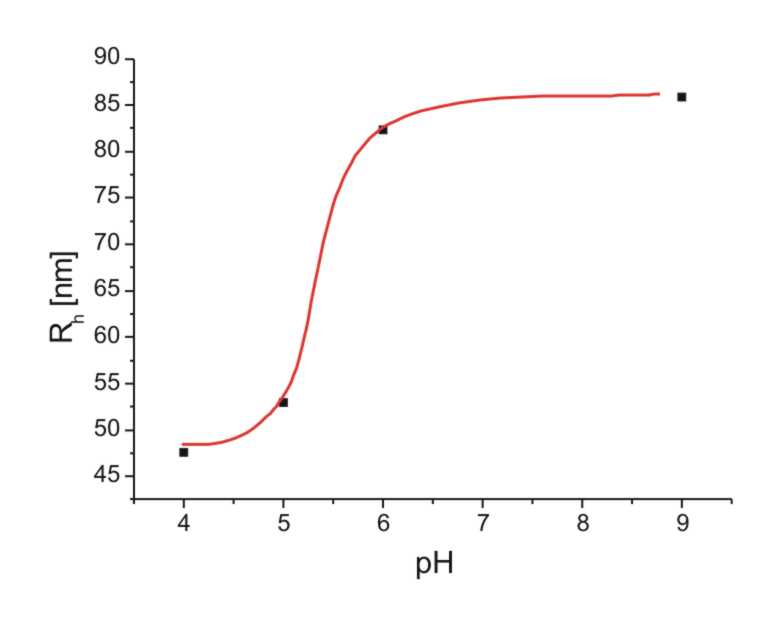
Scheme 3/4: Influence of salt concentration (left, pH=9, Φ=90°, PIB75PMAA1590) and of pH-value (right, c(NaCl)=0.1M, Φ=90°, PIB75PMAA1590) on size of aggregates
With Cryo-TEM-technique the spherical core-corona-structure of the micelles can also be seen (scheme 5).Up to now, first tries to see the core-shell-corona-structure of the aggregates after addition of P4VPQ did not succeed.
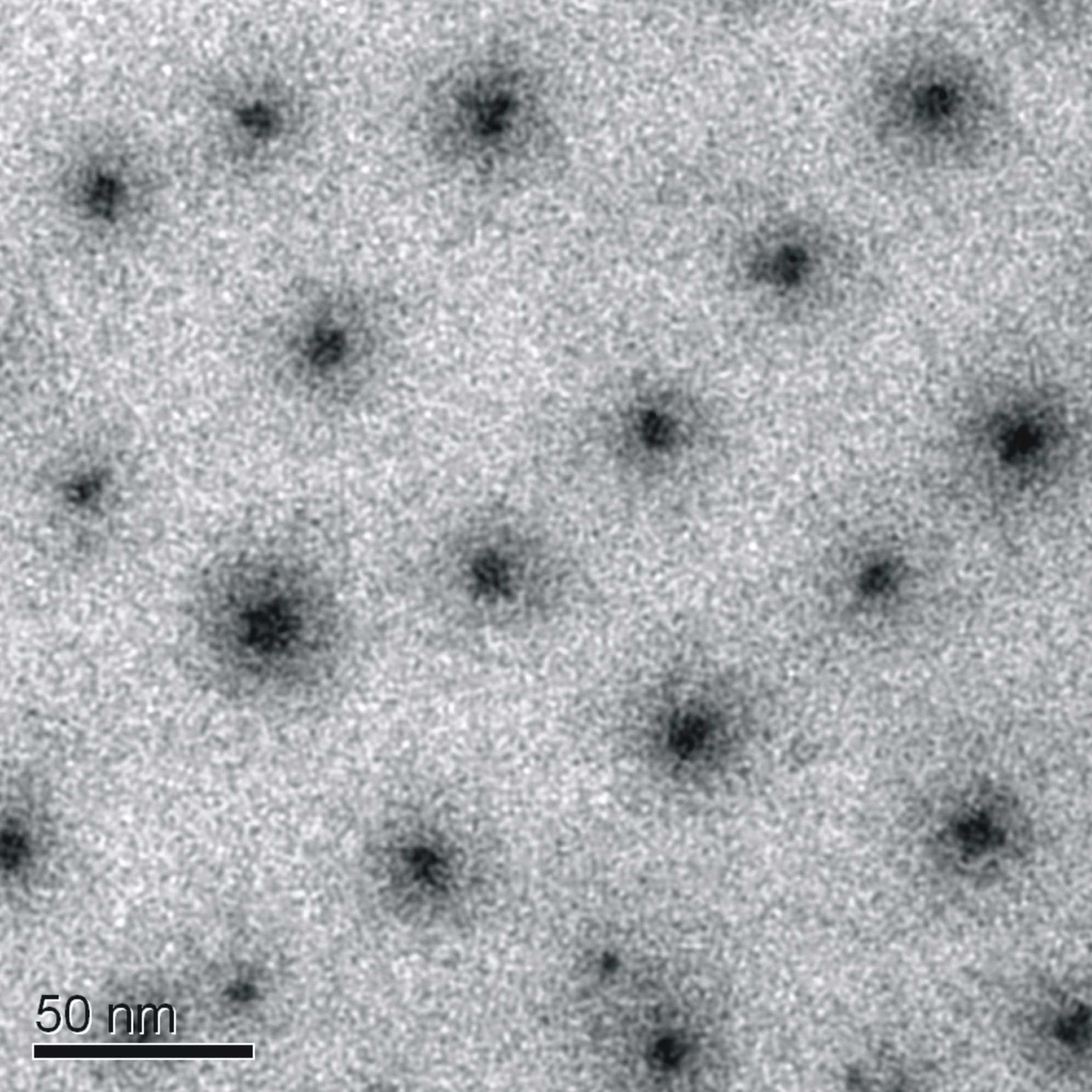
Scheme 5: Cryo-TEM of 1 wt-% solution of PIB31PMAA171 with DN=1 (CsOH)
A new task is to find a synthetic way to finally obtain linear PIB-b-PAA diblock copolymers. The micellization of such copolymers in aqueous solutions is planned to be systematically investigated. In particular, the pH- and salt-induced structural changes of PIB-b-PAA micelles can be followed by means of SANS, SLS and DLS to be finally compared with the results previously obtained for PIB-b-PMAA micelles in order to reveal a role of nature of ionic block on the formed macromolecular assemblies. The results obtained by means of SANS, DLS and AUC for IPECs based on PIB-b-PMAA micelles will be compared to those obtained for the new polymer.
Literature:
[1] Martinez-Castro, N.; Lanzendörfer, M.; Müller, A.H.E.; Cho, J.C.; Acar, M.H.; Faust, R.: Polyisobutylene stars and polyisobutylene-block-poly(tert-butyl methacrylate) block copolymers by site transformation of thiophene end-capped polyisobutylene chain ends, Macromolecules 36, 6815 (2003)
[2] Pergushov, D.; Remizova, E.V.; Zezin, A.B.; Müller, A.H.E.; Kabanov, V.A.: Novel water-soluble micellar interpolyelectrolyte complexes, J. Phys. Chem. B. 107, 8093 (2003)
[3] Pergushov, D.; Remizova, E.V.; Gradzielski, M.; Lindner, P.; Feldthusen, J.; Zezin, A.B.; Müller, A.H.E.; Kabanov, V.A.: Micelles of Polyisobutylene-block-Poly(Methacrylic Acid) Diblock Copolymers and Their Water-Soluble Interpolyelectrolyte Complexes Formed With Quaternized Poly(4-Vinyl Pyridine), Polymer 45, 367 (2004)
>> Publications
>> Projects
>> PhD Thesis


